Vaccination Registration App for Foreigners Will Be Ready Soon
The government is developing a vaccination registration app, especially for foreign residents, as there has been confusion about the registration processamong expats, migrant workers and other foreigners. Ministry of Foreign Affairs deputy spokesperson Natapanu Nopakun said mass vaccinations could begin as soon as next week, as supplies have arrived earlier…
Thailand Expects to Have up to 6 Million Doses of Moderna COVID-19 Vaccine
Thailand’s Private Hospital Association (PHA) expects 5-6 million doses of Moderna’s COVID-19 vaccine to be shipped to Thailand in the second half of this year. They will be offered as a commercial alternative to AstraZeneca and Sinovac. PHA secretary-general Paiboon Eksaengsri said the association is coordinating with Zuellig Pharma, which…
Thai Food and Drug Administration Confirms Each Country Can Register COVID-19 Vaccines
Thailand’s Food and Drug Administration (FDA) has confirmed that each country has the right to register the COVID-19 vaccines which will be used by their people, without relying on a vaccine list approved by World Health Organization (WHO). FDA Secretary-General Dr Paisarn Dunkum said COVID-19 vaccines, approved under the WHO…
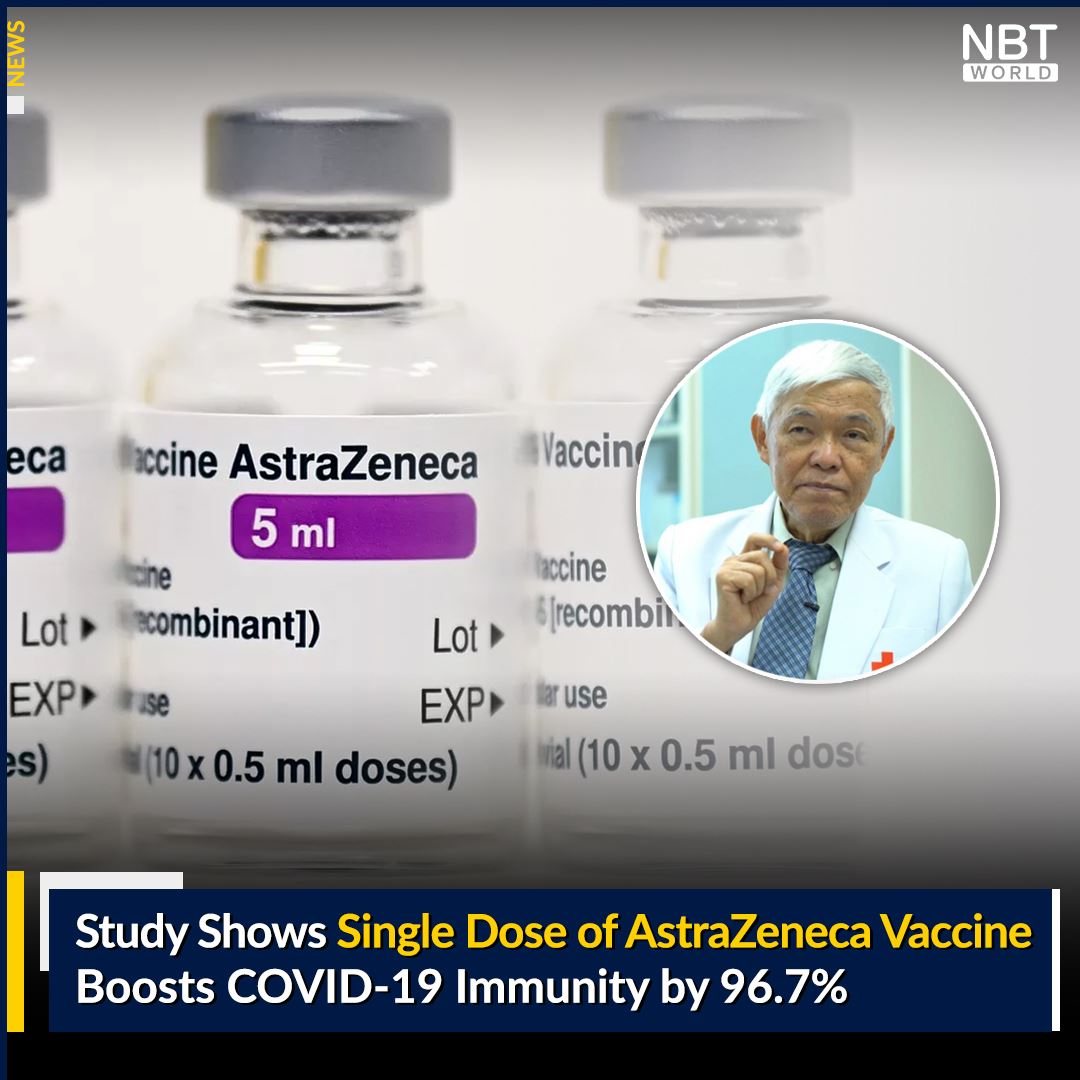
Study Shows Single Dose of AstraZeneca Vaccine Boosts COVID-19 Immunity by 96.7%
A study, conducted by Chulalongkorn’s Center of Excellence in Clinical Virology, shows that those who receive one shot of the AstraZeneca COVID-19 vaccine showed a 96.7% increase in immunity, when compared to unvaccinated individuals. Prof. Dr. Yong Poovorawan said the study also found those who had recovered from COVID-19, between…
FDA approves COVID-19 AstraZeneca vaccine for emergency use
The FDA Secretary General Paisal Dunkhum, said today the FDA had approved the registration of the COVID-19 vaccine produced by AstraZeneca in Italy, after the company had sent nearly 10,000 pages of documents for registration in Thailand for approved use during the state of emergency since December 22, 2020. Efficacy,…
Serum Institute Seeks Emergency Use Nod For Covid-19 Vaccine In India, Second After Pfizer
The SII on Sunday became the first indigenous company to apply to DCGI seeking emergency use authorisation for the Oxford COVID-19 vaccine in the country. The Serum Institute of India on Sunday became the first indigenous company to apply to the Drugs Controller General of India (DCGI) seeking emergency use…